
SOLUTIONS > PROCESS
Audit Management

Streamline audit management
In the life sciences industry, a well-executed audit management process supports compliance, ensures product quality, and reduces risks.

Automate Processes
Automation replaces manual scheduling, tracking, and reporting with predefined workflows that guide auditors and auditees through each stage of the process. Corrective and preventive actions can be initiated directly from audit findings, ensuring accountability and timely resolution. By eliminating manual steps, teams reduce the risk of oversight and free up resources for higher-value quality activities.
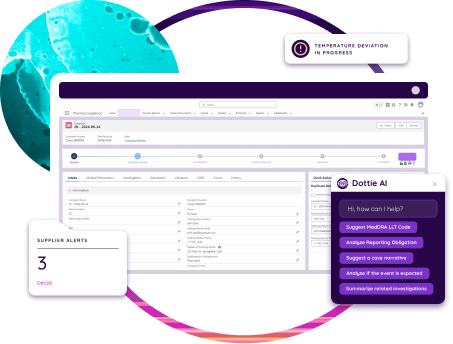
Unify Data
Dot Compliance connects audit records with related data such as CAPAs, training, and supplier management, creating a unified source of truth. This integration supports consistent reporting and faster access to information during inspections. Auditors can review evidence, documentation, and corrective actions in real time, improving accuracy and reducing preparation time.

Enhance Visibility
Real-time dashboards and analytics give quality leaders a complete view of ongoing and completed audits. Trends and recurring issues can be identified quickly, enabling preventive action and continuous improvement. With full visibility and traceability, life sciences organizations can maintain constant audit readiness, strengthen compliance, and ensure product quality across all operations.

Why Dot Compliance Audit Management

The eQMS that keeps you one step ahead
Dot Compliance is the industry’s first AI-powered eQMS, built to get you up and running fast – and to give you the insights to drive quality efficiently.