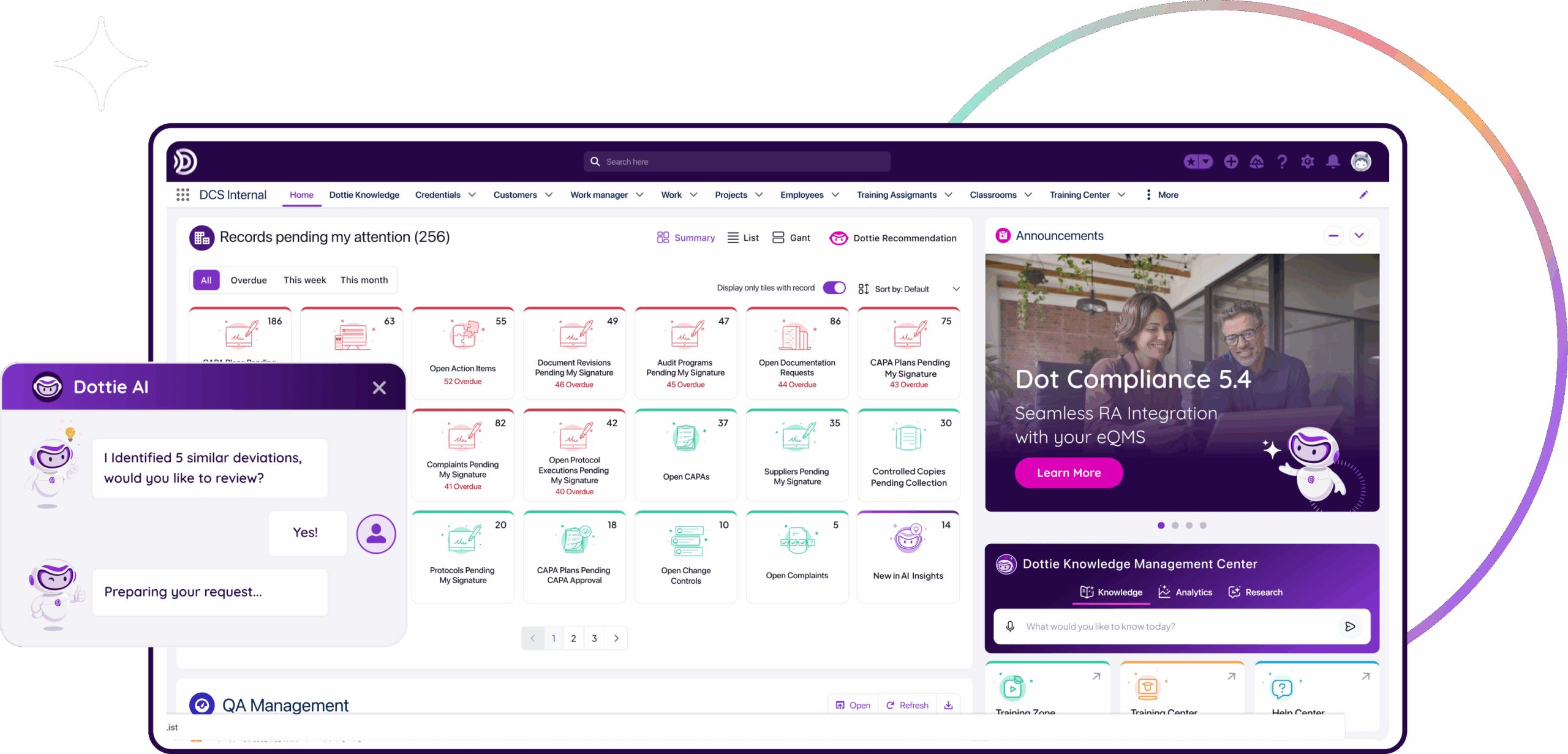
Ensure compliance with an AI-powered eQMS
WHY CHOOSE DOT COMPLIANCE?

AI-powered. Ready-to-deploy.
Move faster with the industry’s first pre-validated, ready-to-deploy eQMS, purpose-built for life sciences. Accelerate your deployment, leverage built-in best practices, and harness AI-powered quality management from day one.

Native to Salesforce
Dot Compliance is 100% Salesforce-native, which means you get a consistent user experience, easy integration with existing systems, and scalability and security right out of the box.
Whether you’re managing one site or many, our architecture grows with you and keeps data and processes aligned across your organization.

Trusted by life sciences
Purpose-built for pharma, biotech, and medical device companies, Dot Compliance streamlines quality processes, simplifies regulatory compliance, and accelerates time to market.
Chosen by teams like yours
From fast-growing biotech startups to global pharmaceutical leaders, life sciences companies choose Dot Compliance to modernize their quality systems.




Built by people who’ve lived it
Dot Compliance is more than software. It’s experience, embedded. Our team includes former quality and compliance leaders who’ve worked across the life sciences. That real-world knowledge is apparent in all aspects of our solutions.
“Dot Compliance has resulted in improved efficiency, giving us greater control, and has enabled us to make data-driven decisions.”
FAQs
Do I need Salesforce to use Dot Compliance?
Is Dot Compliance validated?
Can I start small and scale later?
What support is available after go-live?
How does Dot Compliance support life sciences companies?
How does Dot Compliance ensure fast implementation?
Can Dot Compliance integrate with existing systems?
Global compliance, baked in
We’re built for regulated industries, not retrofitted for them.
Dot Compliance is certified with:
- ISO/IEC 42001
- ISO 27001
- ISO 9001
- ISO 27017
All of our solutions support ISO 13485 and
ISO 14971 and are fully compliant with:
- 21 CFR Part 11
- 21 CFR Part 820
- EU Annex 11
The ready-to-use QMS software that delivers speed and smarts
Used by life sciences organizations around the world, Dot Compliance empowers teams to manage quality with confidence, maintain continuous compliance, and keep every process inspection ready.