The Dot Compliance Blog
Your source for AI, quality, and compliance updates, insights and best practices across the life sciences industry.
All
21 CFR Part 11AnnouncementArtificial IntelligenceAudit ManagementBest PracticesBiotechnologyCAPAChange ManagementClinicalComplaint ManagementCosmeticsData and AnalyticsDocument ManagementeQMSISO 13485Life SciencesManufacturingMedical DevicePharmaceuticalQuality 4.0Regulatory ComplianceRisk ManagementSaaSTraining Management

Can an eQMS Help with Regulatory Submissions and Inspections?
August 2025

Why Are More Life Sciences Companies Moving to SaaS-Based QMS Platforms?
August 2025

How Does an eQMS Help Maintain GxP Compliance?
August 2025

What Does ISO 13485 Require from an eQMS for Medical Devices?
August 2025

How Is an eQMS Used in Biotech vs. Med Devices?
August 2025

Which Features Matter Most When Choosing an eQMS?
August 2025

How Is AI Changing the Way Life Sciences Companies Manage Quality?
July 2025
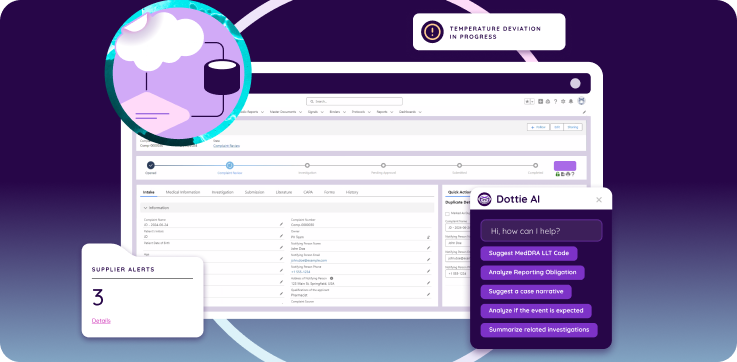
How Should a QMS Be Configured for GxP Compliance?
July 2025

Can a QMS Help Us Prepare for an FDA Inspection?
July 2025

What Documentation Does a QMS Need for FDA 21 CFR Part 11?
July 2025

Can a QMS Help You Pass an FDA Audit?
July 2025

Why Is an eQMS Critical for Life Sciences Companies in 2025?
July 2025