The Dot Compliance Blog
Your source for AI, quality, and compliance updates, insights and best practices across the life sciences industry.
All
21 CFR Part 11AnnouncementArtificial IntelligenceAudit ManagementBest PracticesBiotechnologyCAPAChange ManagementClinicalComplaint ManagementData and AnalyticsDocument ManagementeQMSISO 13485Life SciencesManufacturingMedical DevicePharmaceuticalQuality 4.0Regulatory ComplianceRisk ManagementSaaSTraining Management

5 Considerations for an eQMS in Life Sciences
December 2021

Women in Leadership: Making Business Better
November 2021

How Women in the Workforce Impact Quality
November 2021

Quality Culture: How your QMS Should be Built into its Foundation
November 2021

Top 10 Medical Device Testing Companies of 2021
November 2021

Connecting the Dots in Life Sciences: Part 4 – Electronic Batch Records
November 2021
6 Steps to HIPAA Risk Assessment Success
November 2021

Connecting the Dots in Life Sciences: Part 3 – Binders
October 2021
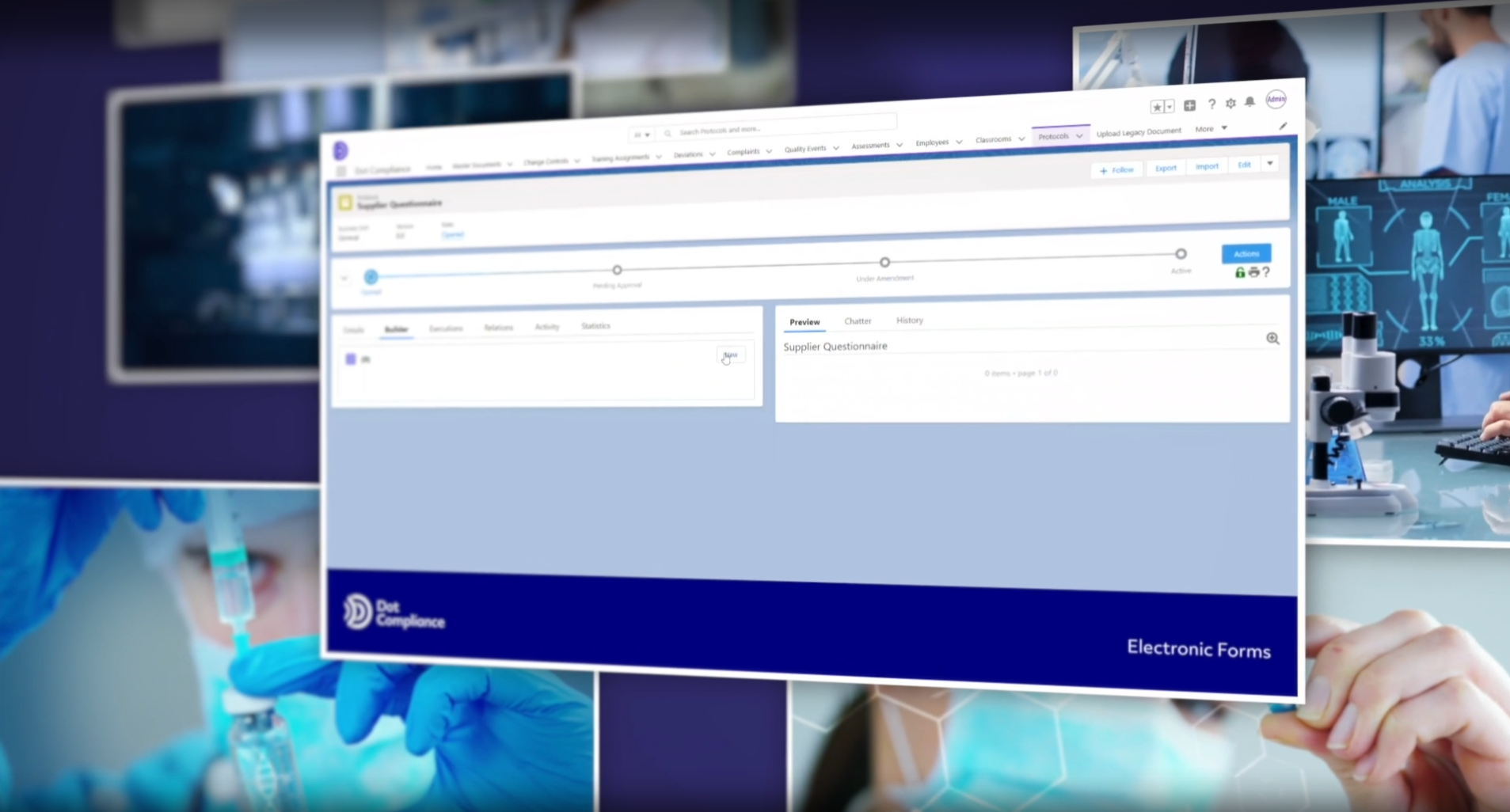
Connecting the Dots in Life Sciences: Part 2 – Electronic Forms
October 2021

Top 8 Change Management Tools
October 2021

MDR: Maximizing Changes to Medical Device Reporting Regulations
October 2021

The Medical Cannabis Industry and Compliance
October 2021