Deviations/Non-conformance Management
Managing deviations and other quality events is required by GMP, but properly capturing and measuring these events is at the core of quality improvements regulators will demand from your organization as they renew their focus on quality. Dot Compliance cloud-based, fully validated QMS provides organizations with the ability to manage deviations so they are identified and recorded. This includes Incidents that could affect the quality or the reliability of records or tests to be investigated and resolved.
Automate the step-by-step process
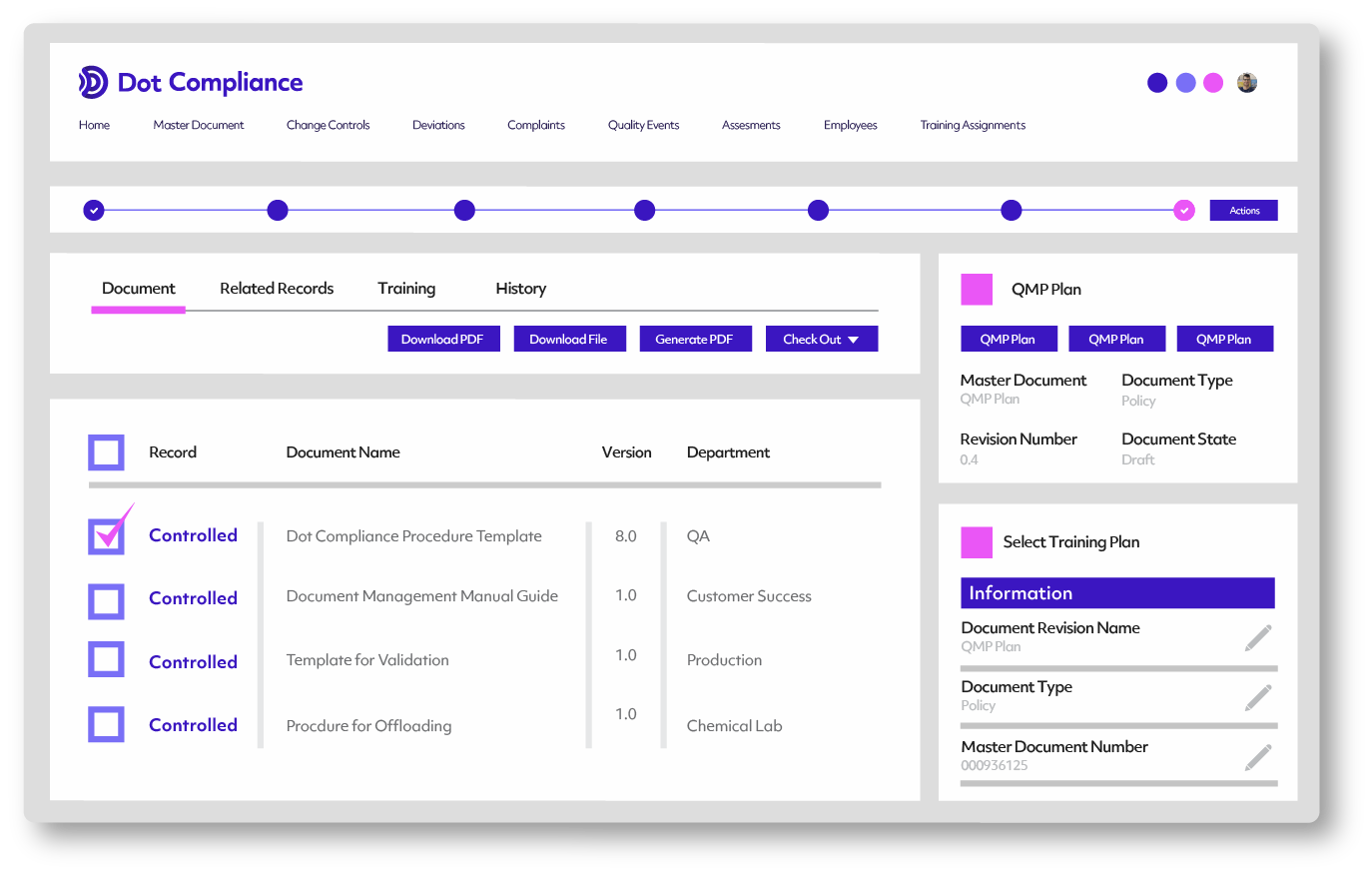
Dot Compliance QMS system provides pre-defined reports for initiating any possible deviation investigation. The entire deviation process is supported by the system, from initiation and investigation, to review, approval and closure in compliance with CFR 21 Part 11.

Identify trends in your quality data
Collecting quality information through investigations helps organizations put together intelligent information which supports improvements in existing processes, higher operational efficiency and lower re-occurrence of deviations.
Allows visibility across the quality system to track and trace all activities
Complete audit trail that supports compliance with part 11 requirements. The system also supports e-signatures, data integrity, and data security for better compliance.

Easily integrate with other quality processes
Harmonize the entire quality management process by easily integrating the deviation software with other quality processes and systems like CAPA, Change Control, Complaints, etc. Dot Compliance contributes to the continuous improvement of quality.

See Dot Compliance in Action
Book a demo to see our ready to use solutions for yourself.


