Regulatory Affairs Solution
Streamlined regulatory and quality processes that simplify submissions, accelerate time to market, and ensure compliance.
Complete and Cost effective Regulatory Affairs Process Automation.
Dot Compliance’s regulatory information management solution breaks down the traditional silioed approach of communication with regulatory agencies and personnel. Manage all regulatory data and documentation, registration of products, product variation and registration renewals in one system. Integration with document control and other system capabilities provides flexible, complete, and cost-effective RA process automation.
Improved collaboration
Automate regulatory and quality processes under one system. Solution is compliant with strict industry standards such as 21 CFR Part 11. Document control integration includes validation package and project validation services.
Real-time communication
Track the entire product lifecycle with advanced metric and KPI reporting through easy to use dashboards.
Streamlined global submissions
Compliance with regulatory bodies, supporting correspondences with regulatory agencies in any given country.

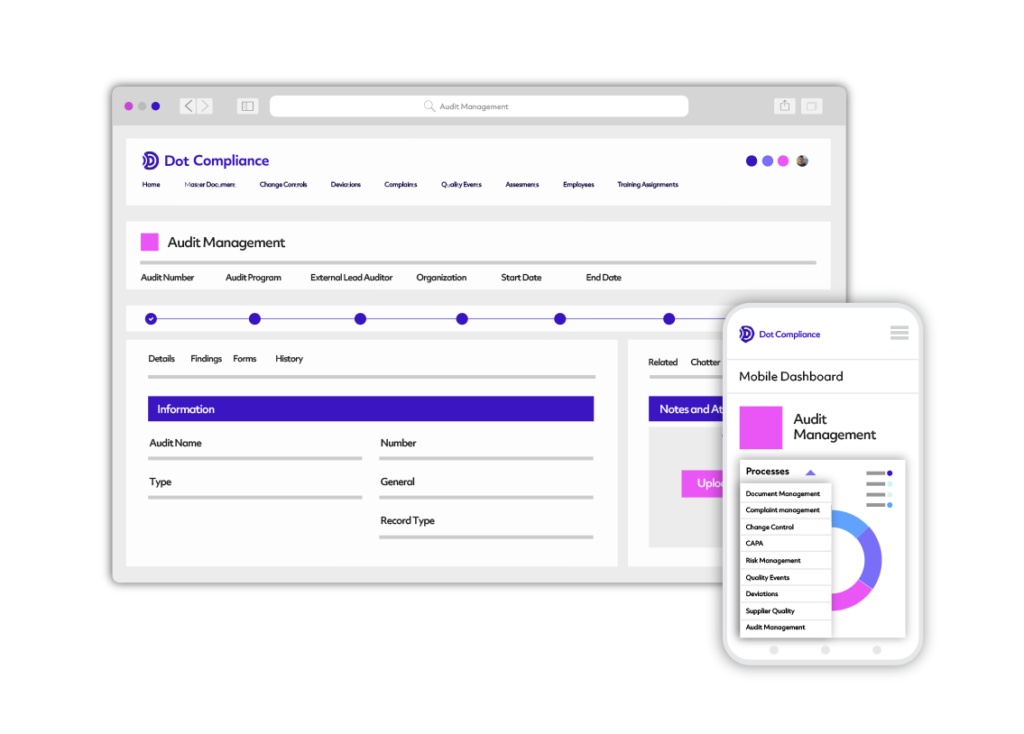
See Dot Compliance in Action
Book a demo to see our ready to use solutions for yourself.


